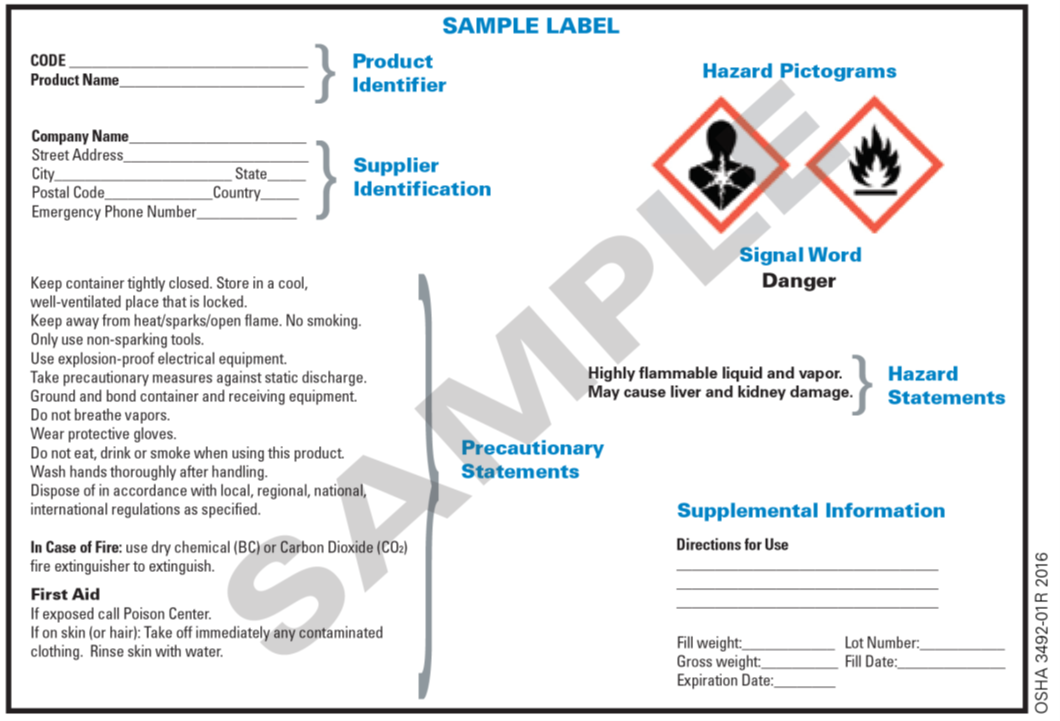
42 regulations require that product labels on containers
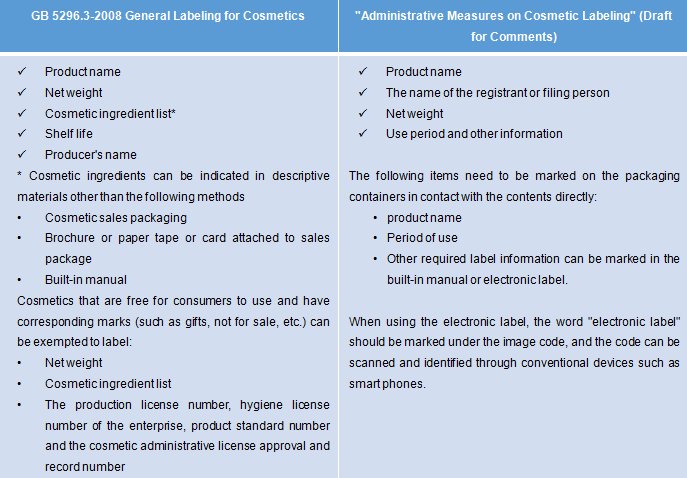
Industry Guide for the labelling of cosmetics - Canada.ca Section 2 of the Consumer Chemicals and Containers Regulations, ... 7.2.2 Products that have both outer and inner labels. If a product in a pressurized metal container has both an ... The Cosmetic Regulations require that all cosmetic products sold in Canada must list the ingredients on the label using the INCI labelling system as found ... Cosmetics Labeling Guide | FDA Regulations require that "[the label of a cosmetic product shall bear a warning statement whenever necessary or appropriate to prevent a health hazard that may be associated with the product" [21 ...
CBD Gummies | CBD Infused Gummies | 100% Vegan While this takes the CBD a bit longer to kick in than, say, a CBD vape product, the benefit of consuming a CBD edible is that the effects can last as long as six to eight hours. But be patient, CBD gummies, softgels and other edibles can take 30 minutes to two hours to fully kick in, depending on bioavailability factors , such as height, weight, body mass, metabolism, and CBD …
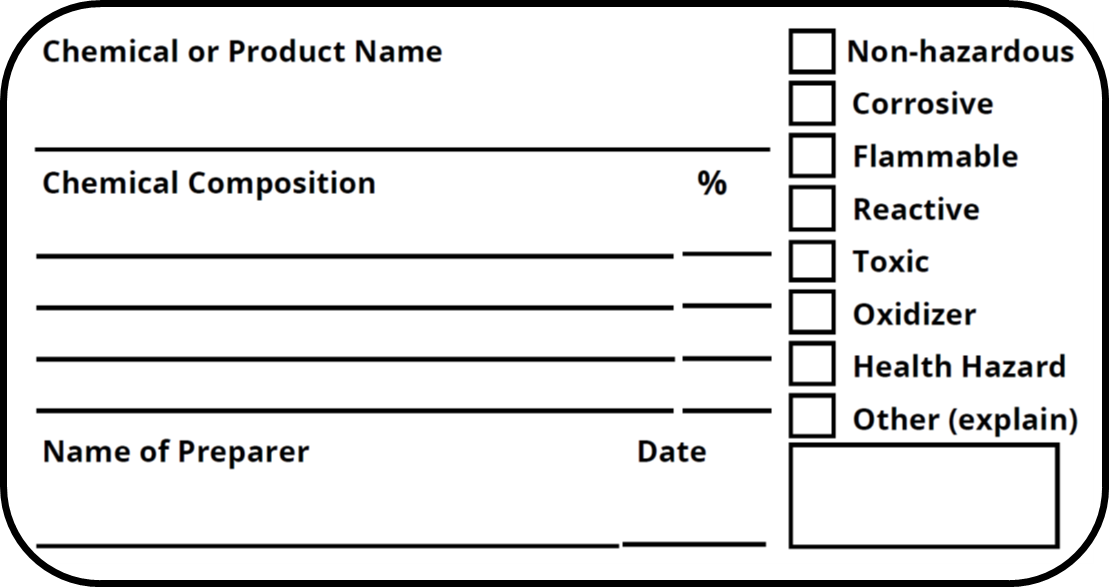
Regulations require that product labels on containers
Federal Register :: National Bioengineered Food Disclosure Standard 21.12.2018 · A multi-ingredient product that contained one of these as the second most predominant ingredient or lower, could also require disclosure, unless the product is otherwise exempt (for example, due to the predominance of another ingredient such as chicken or beef, as described above). C. Bioengineered Food California Code of Regulations, Title 8, Section 5193. Bloodborne ... 5. Red bags or red containers may be substituted for labels except for sharp containers or regulated waste red bags. Bags used to contain regulated waste shall be color-coded red and shall be labeled in accordance with subsection (g)(1)(A)2. Labels on red bags or red containers do not need to be color-coded in accordance with subsection (g)(1)(A)3. CFR - Code of Federal Regulations Title 21 - Food and Drug … 20.7.2022 · (ii) For all other food products, the product shall be eligible for an exemption for any 12-month period if, for the preceding 12 months, the person claiming the exemption employed fewer than an average of 100 full-time equivalent employees and fewer than 100,000 units of that product were sold in the United States, or in the case of a food product that was not sold in the …
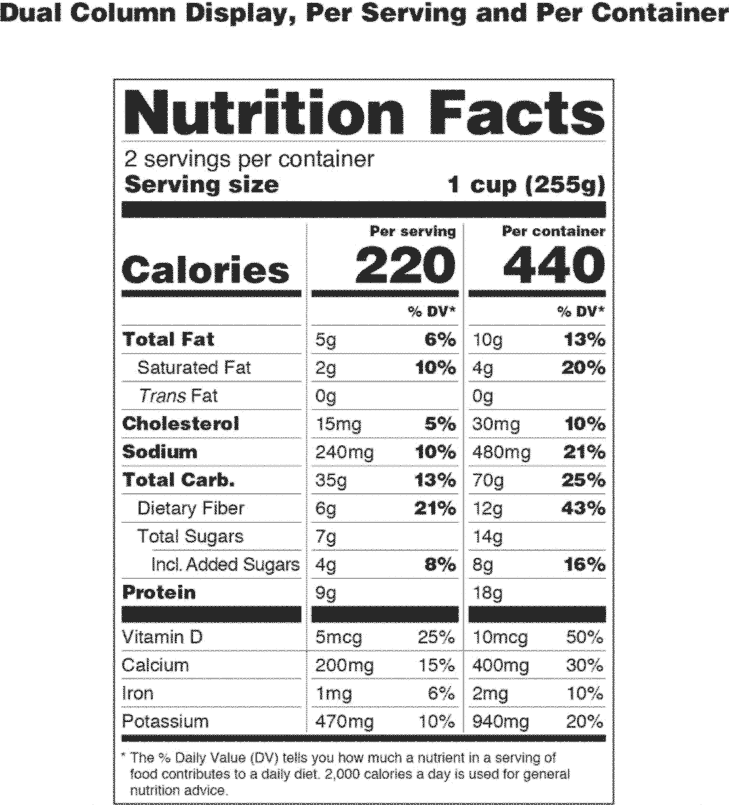
Regulations require that product labels on containers. 1910.1030 - Occupational Safety and Health Administration Red bags or red containers may be substituted for labels. 1910.1030(g)(1)(i)(F) Containers of blood, blood components, or blood products that are labeled as to their contents and have been released for transfusion or other clinical use are exempted from the labeling requirements of paragraph (g). Changes to the Nutrition Facts Label | FDA 7.3.2022 · Spanish (Español) The Nutrition Facts label on packaged foods was updated in 2016 to reflect updated scientific information, including information about the link between diet and chronic diseases ... Packaging and labeling - Wikipedia The purposes of packaging and package labels. Packaging and package labeling have several objectives. Physical protection – The objects enclosed in the package may require protection from, among other things, mechanical shock, vibration, electrostatic discharge, compression, temperature, etc. Regulations Amending the Natural Health Products Regulations: … Jul 06, 2022 · In this analysis, businesses will either continue with existing labelling configurations, footnote 59 or change to use a different labelling configuration, such as peel-back labels, or choose to adopt outer boxes, inserts or leaflets. footnote 60 No product discontinuation is assumed because these amendments do not require any product to stop ...
California Code of Regulations, Title 8, Section 5194. Hazard ... Sep 28, 2018 · (B) Any food, food additive, color additive, drug, cosmetic, or medical or veterinary device, including materials intended for use as ingredients in such products (e.g., flavors and fragrances), as such terms are defined in the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 301 et seq.) and regulations issued under that Act, when they are subject to the labeling requirements of that Act and ... CFR - Code of Federal Regulations Title 21 - Food and Drug … 20.7.2022 · (ii) For all other food products, the product shall be eligible for an exemption for any 12-month period if, for the preceding 12 months, the person claiming the exemption employed fewer than an average of 100 full-time equivalent employees and fewer than 100,000 units of that product were sold in the United States, or in the case of a food product that was not sold in the … California Code of Regulations, Title 8, Section 5193. Bloodborne ... 5. Red bags or red containers may be substituted for labels except for sharp containers or regulated waste red bags. Bags used to contain regulated waste shall be color-coded red and shall be labeled in accordance with subsection (g)(1)(A)2. Labels on red bags or red containers do not need to be color-coded in accordance with subsection (g)(1)(A)3. Federal Register :: National Bioengineered Food Disclosure Standard 21.12.2018 · A multi-ingredient product that contained one of these as the second most predominant ingredient or lower, could also require disclosure, unless the product is otherwise exempt (for example, due to the predominance of another ingredient such as chicken or beef, as described above). C. Bioengineered Food














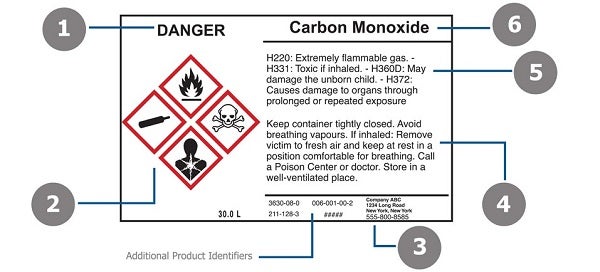

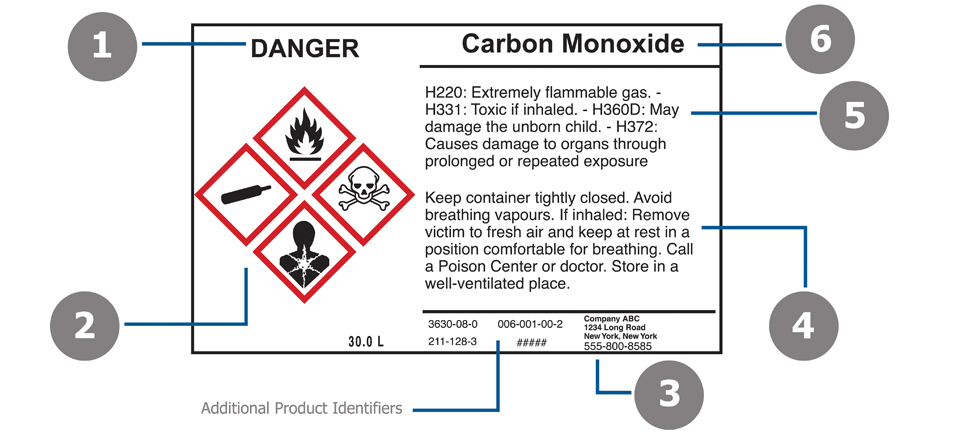


















Post a Comment for "42 regulations require that product labels on containers"